20+ ionization energy calculator
A mass spectrometer can. Lets consider for hydrogen The first orbit energy 218 10 18 Jatom or 13123.

Ion Energy Gets 3 6 Million In Pre Series A Funding Round The Economic Times
The Ionization energy in KJ mole formula is defined as the minimum amount of energy required to remove the most loosely bound electron of an isolated neutral gaseous atom or molecule is.

. Please note that the elements do not show their natural relation towards each other as in the Periodic system. Divide the speed of light approximately 300000000 ms by the wavelength to get the. It also compares the Subtraction method to the Koopmans Method.
There you can find the metals. Hydrogen Energy Levels Calculator. 105 rows If you need to convert ionization energy between above or less common units you can use our another calculator.
C 2 H 4 O. See section IB1 for a periodic table view. Mostly atoms with atomic number less than than 36 Krypton except for most of the transition metals.
Calculate the ionization energy eqrm E_i eq per atom in SI units. With this handy calculator determining the energy level of an atom is no longer difficult. The energy essential to take away an electron from a gaseous atom A or a gaseous molecule AB is titled as ionization energy.
Referring to the following equation. To calculate photon energy from wavelength. Science Chemistry calculate the ionization energy for a hydrogen like atom with one electron and Z5 from the ground state.
119 rows The unity for ionization energy is eV. Substitute the value of ionization energy from Step 1 in the energy-wavelength formula. Ionization energy chart of all the elements is given belowFirst ionization energy second ionization energy as well as third ionization energy of the.
Ionization energy for the removal of an electron from a neutral atom can be calculated by substituting the orbit number of the electron before transition as. First Ionization Energy of Beryllium is 93226 eV. 315g Libr 350 H₂0 g₁ x T I molli Be 879 LiBr 87 g 17665600J.
To obtain the correct energy level in a short period of time you must. Six or fewer heavy. The energy needed to detach one electron.
C 3 H 5. This script calculates the ionization eneregy of your atom. In the Input section you will enter the ion energy value.
Ionization energy also called ionization potential is the energy necessary to remove an electron from the neutral atom. Make sure your wavelength is in meters. Numerically we describe ionization energy as the orbital energy of the electron with the reverse sign.
Species in the CCCBDB.

Ionization Energy Periodic Table Trends Chemtalk

Analytical Gradients For Nuclear Electronic Orbital Time Dependent Density Functional Theory Excited State Geometry Optimizations And Adiabatic Excitation Energies Journal Of Chemical Theory And Computation

Calculations Ionization Energies Ewt
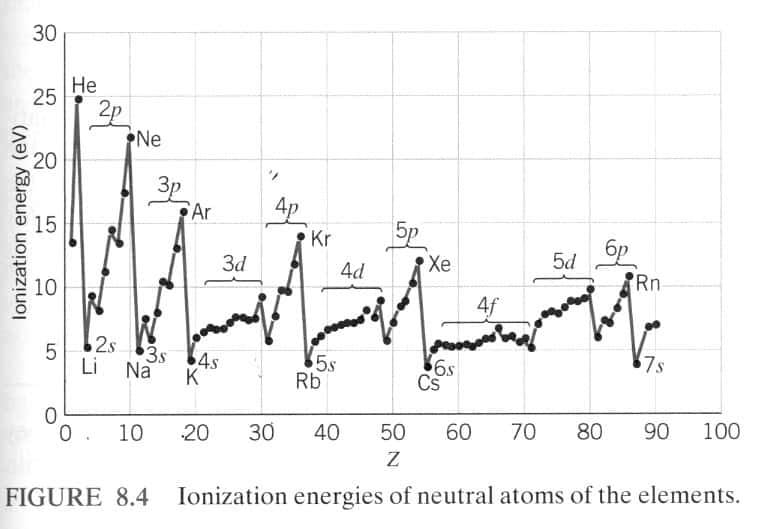
Ionization Energy Schoolworkhelper

Color Online A Definition Of Various Ionization And Dissociation Download Scientific Diagram
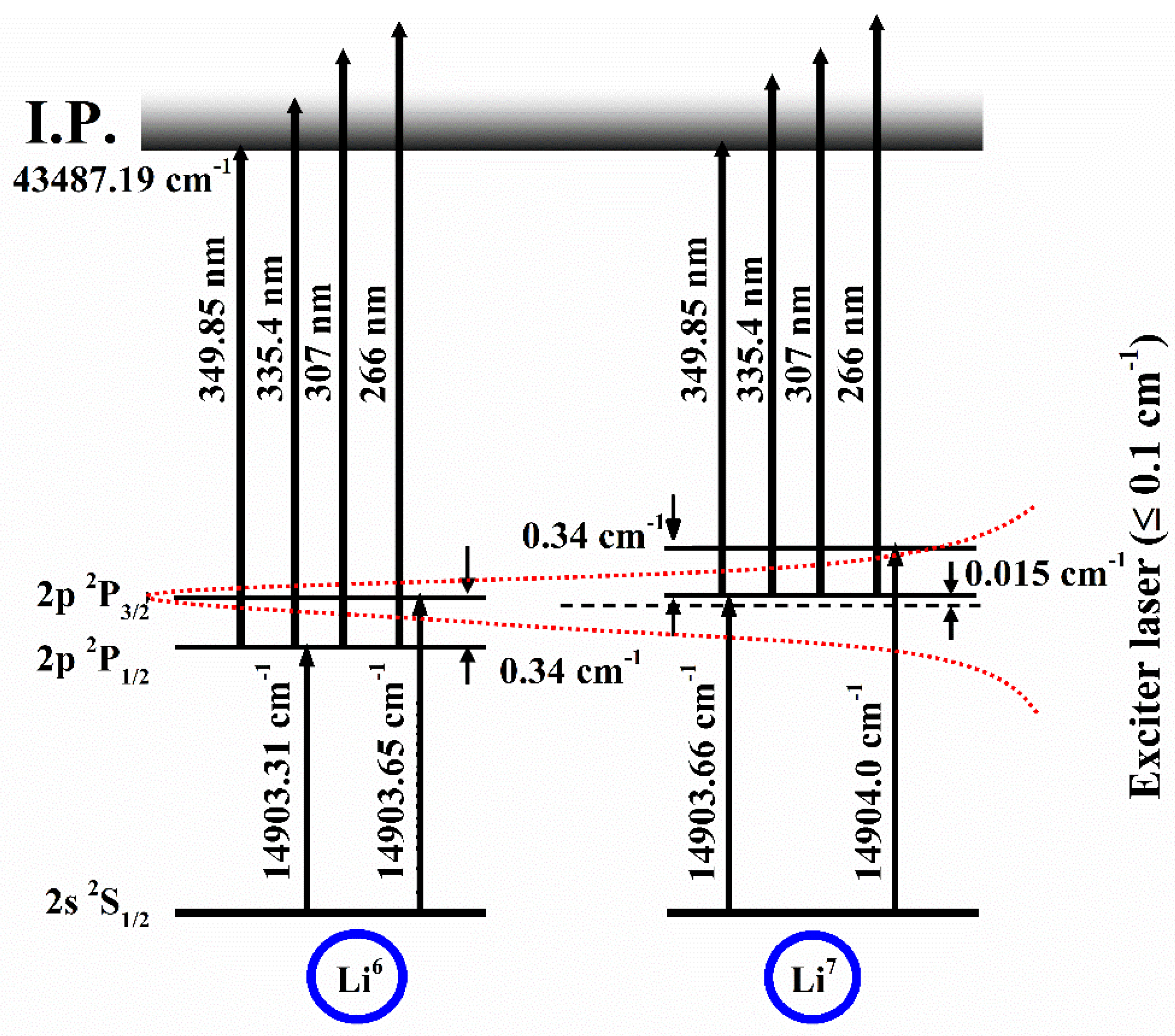
Atoms Free Full Text Measurement Of Photoionization Cross Section For The Excited States Of Atoms A Review Html
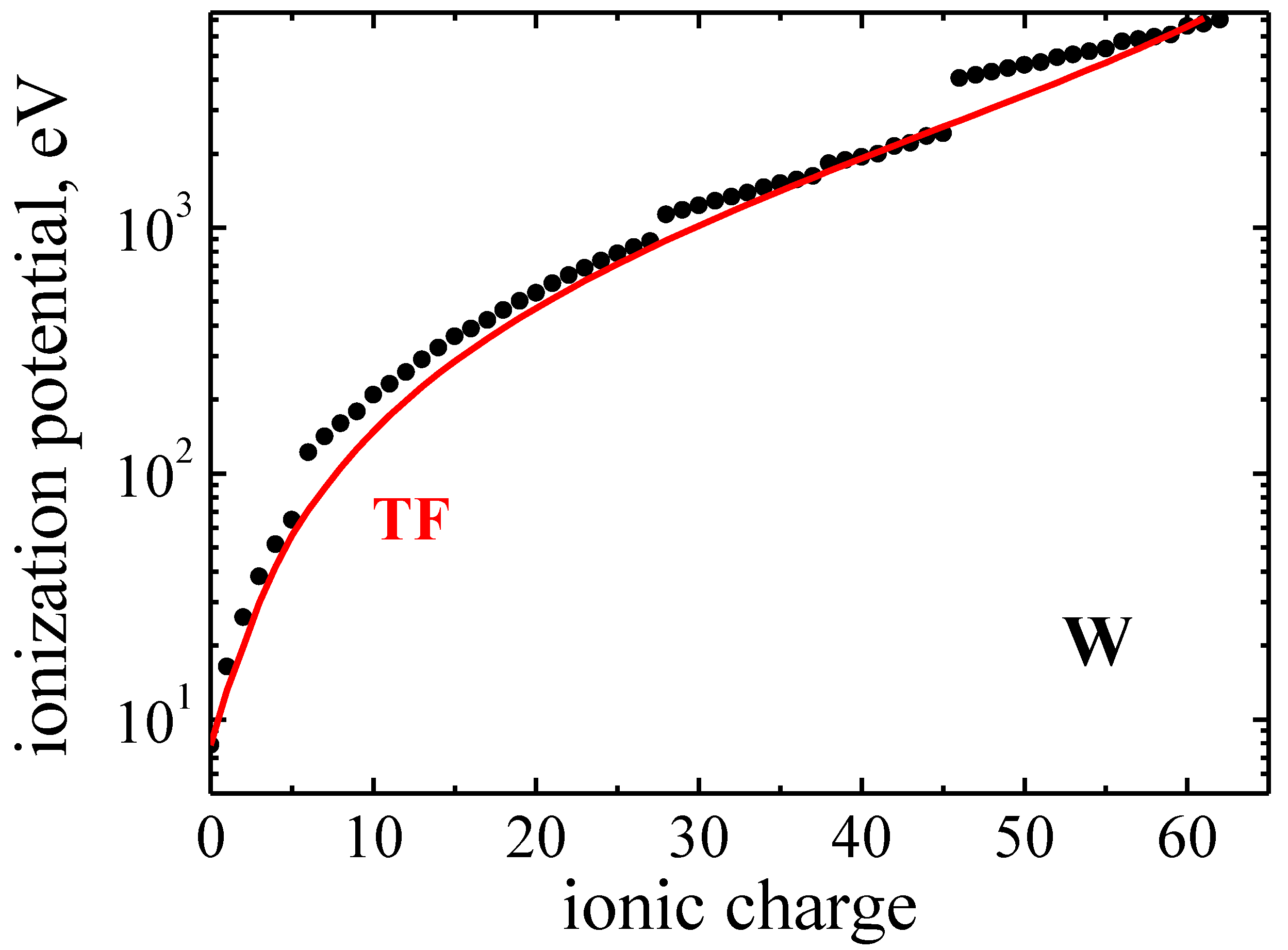
Atoms Free Full Text Tungsten Ions In Plasmas Statistical Theory Of Radiative Collisional Processes Html

Ionization Potential Depression In Dense Iron Plasmas Near Solid Density Sciencedirect
Why Is Ionisation Energy In A Gaseous Phase Quora
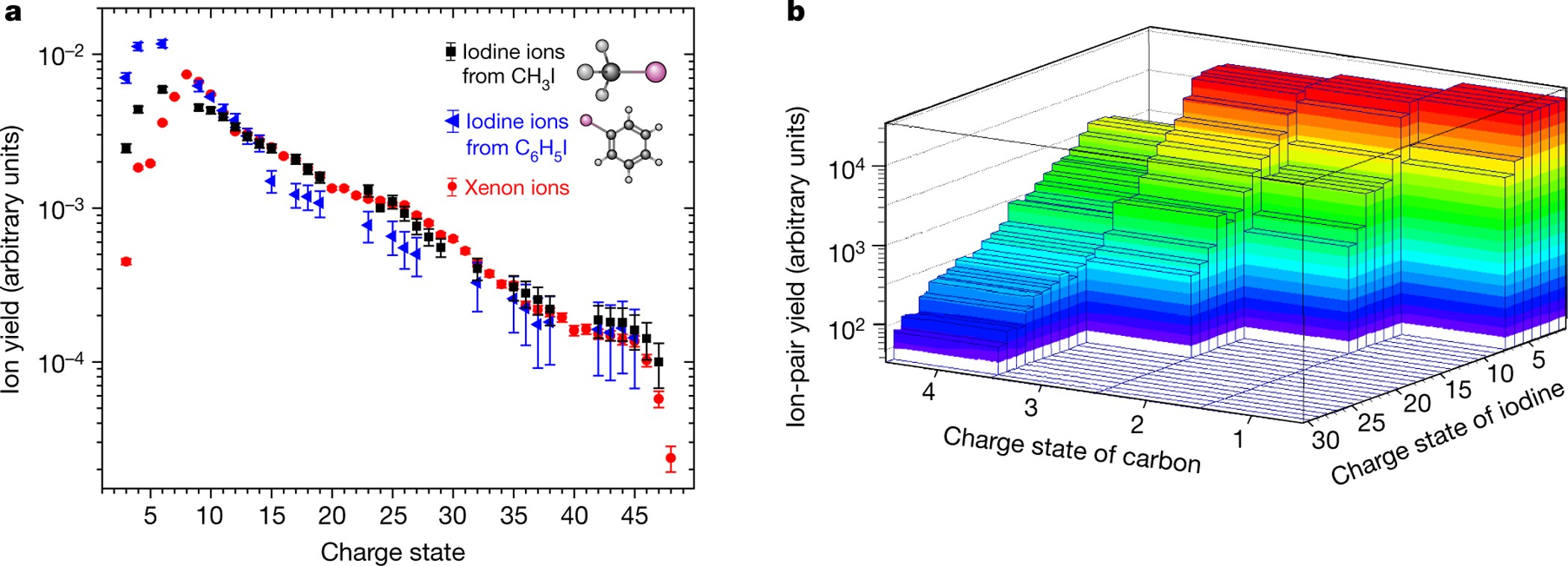
Femtosecond Response Of Polyatomic Molecules To Ultra Intense Hard X Rays Nature

Dissociation And Multiple Ionization Energies For Five Polycyclic Aromatic Hydrocarbon Molecules Semantic Scholar

Ionization Energies Of Highly Charged Tungsten Ions And Overview Of Download Scientific Diagram
Ionization Energy Periodic Table Trends Chemtalk
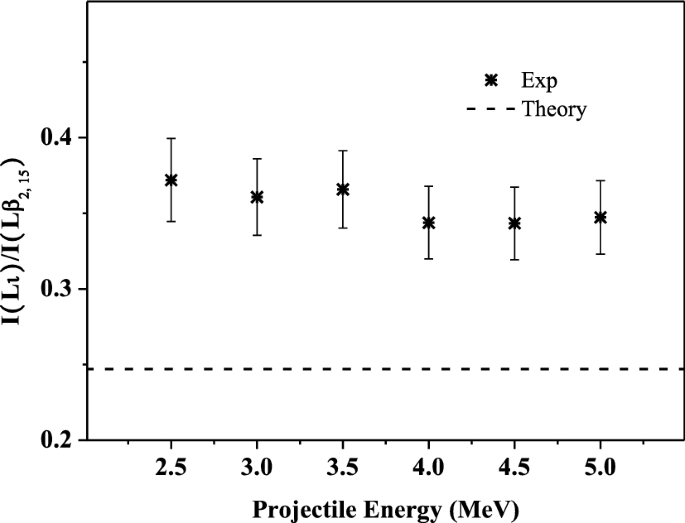
Multiple Ionization Of Iodine For 2 5 5 0 Mev I22 Ions Impacting On Fe Target Scientific Reports

The Parts Of The Periodic Table

Pdf Methods Of Calculating Ionization Energies Of Multielectron Five Or More Isoelectronic Atomic Ions

Dissociation And Multiple Ionization Energies For Five Polycyclic Aromatic Hydrocarbon Molecules Semantic Scholar